Cannabinoids for Medical Use a Systematic Review and Meta-analysis
- Inquiry commodity
- Open up Access
- Published:
Therapeutic apply of cannabis and cannabinoids: an evidence mapping and appraisement of systematic reviews
BMC Complementary Medicine and Therapies book 20, Article number:12 (2020) Cite this article
Abstract
Background
Although cannabis and cannabinoids are widely used with therapeutic purposes, their claimed efficacy is highly controversial. For this reason, medical cannabis utilize is a broad field of research that is rapidly expanding. Our objectives are to identify, characterize, appraise, and organize the current bachelor evidence surrounding therapeutic apply of cannabis and cannabinoids, using evidence maps.
Methods
We searched PubMed, EMBASE, The Cochrane Library and CINAHL, to place systematic reviews (SRs) published from their inception up to December 2017. Two authors assessed eligibility and extracted data independently. We assessed methodological quality of the included SRs using the AMSTAR tool. To illustrate the extent of use of medical cannabis, we organized the results according to identified PICO questions using bubble plots respective to different clinical scenarios.
Results
A total of 44 SRs published betwixt 2001 and 2017 were included in this show mapping with information from 158 private studies. We extracted 96 PICO questions in the following medical atmospheric condition: multiple sclerosis, motility disorders (due east.g. Tourette Syndrome, Parkinson Disease), psychiatry conditions, Alzheimer disease, epilepsy, acute and chronic pain, cancer, neuropathic pain, symptoms related to cancer (e.yard. emesis and anorexia related with chemotherapy), rheumatic disorders, HIV-related symptoms, glaucoma, and COPD. The bear witness almost these atmospheric condition is heterogeneous regarding the conclusions and the quality of the individual primary studies. The quality of the SRs was moderate to loftier according to AMSTAR scores.
Conclusions
Evidence on medical uses of cannabis is broad. However, due to methodological limitations, conclusions were weak in most of the assessed comparisons. Evidence mapping methodology is useful to perform an overview of available enquiry, since it is possible to systematically depict the extent and distribution of show, and to organize scattered data.
Groundwork
Medical cannabis refers to the use of cannabis or cannabinoids for the handling of a medical condition or to alleviate its associated symptoms [one, 2]. The spectrum of substances categorized as medical cannabis include: 1) Phytocannabinoids, which are plant in cannabis herb and resins, east.m. Tetrahydrocannabinol (THC) and Cannabidiol (CBD); 2) Purified cannabinoids which originate from cannabis extracts (e.g. Nabiximols and purified cannabidiol); and 3) Synthetic cannabinoids (e.1000. Dronabinol and Nabilone) [2, 3].
Cannabis sativa produces more 100 phytocannabinoids and the biosynthesis of these substances depends on genomic background and specific environmental weather condition [4]. Additionality, in humans, the utilise of C. sativa has shown a myriad of heterogeneous central and peripheral effects due to endocannabinoid system, whose receptors are scattered throughout the body. The being of many molecules, which possibly attune endocannabinoid system, complicates the scenario [v]. Currently, these are the reasons why, research on C. sativa is complex and difficult.
The history of the use of cannabis for medical purposes is long, every bit these plants have been used for therapeutic purposes for more than 4000 years [6]. Yet, cannabis has a high-risk profile and its medical utilize is highly controversial, fifty-fifty for therapeutic reasons. Despite the adverse furnishings of cannabis use such as risk of developing cannabis dependence, exacerbation of cardiovascular illness, atmospheric precipitation of psychotic disorders [7], and criticism to the bear witness supporting its utilize for medical conditions, several governments accept authorized the medical apply of marijuana in countries such as Canada, the Czech Commonwealth, Federal republic of germany, Italy, the Netherlands, and 23 Us states [eight,9,ten].
To approve the medical use of cannabis, well-designed and statistically powered clinical trials are necessary to investigate patient response [11]. Research on therapeutic uses of cannabis have restrictions due to limitations in gaining access to the quantity, quality, and type of cannabis product necessary to address specific research questions on health effects. There are notable research challenges, such equally the vast spectrum of chemic substances considered as medical cannabis, the lack of dose standardization, and the lack of consensus about medical conditions for which cannabis have been approved. Prove near the benefits and harms related to cannabis utilize is rapidly irresolute, making it difficult to identify and summarize findings in order to make informed decisions and establish research needs.
Evidence mapping is a useful methodology to overview available research about broad knowledge areas. This methodology is useful to systematically draw the extent and distribution of show and to identify gaps for further research. This approach identifies if there is enough evidence to support policy maker'south decisions and to recognize research-dense areas where systematic reviews can exist conducted, every bit well as research questions which should exist prioritized in those fields.
The aim of this evidence mapping is to identify, characterize, assess, and organize the currently available evidence nearly the therapeutic utilize of cannabis and cannabinoids through systematic reviews. Our approach aims to identify the clinical questions most efficacy of medical cannabis assessed in the scientific literature, as well every bit to give an overview well-nigh their potential benefits and harms.
Methods
We followed the approach of the Global Evidence Mapping Initiative [12] with additional components introduced by Ballesteros et al. [13,14,fifteen]. We established these criteria a priori in a protocol (available on request). This show map involved three stages:
Systematic search strategy and selection of relevant studies
We used systematic reviews (SRs) as a comprehensive source of appraised prove. We divers medical cannabis equally the apply of cannabis or cannabinoids to treat a medical status or to alleviate its symptoms. Thus, we based this bear witness mapping in SRs assessing medical cannabis efficacy, effectiveness or safety. We decide to include cannabis and cannabinoids every bit our objective is to place all the bachelor evidence related to medical cannabis, still cannabis and their isolated compounds could have different pharmacological properties and efficacy profiles. We considered SRs that conducted a search in at to the lowest degree two databases, and that appraise the quality or risk of bias of the included studies.
We excluded SRs focused on toll-effectiveness only. Additionally, we excluded SRs assessing Rimonabant (i.e. a synthetic cannabinoid studied for weight control) since information technology acts every bit a functional antagonist of cannabinoids receptor [16].
We collected central search terms from previous reviews and SRs on medical cannabis by using natural and MeSH terms. We searched in PubMed, EMBASE, The Cochrane Library and CINAHL, from their inception upward to Dec 2017. There were no language restrictions.
We reviewed references in relevant articles to identify potential boosted reviews. Search strategies are reported in Additional file 1.
Later duplicates were eliminated, two reviewers independently screened titles and abstracts (NMO, SNG) of the retrieved references and determined their relevance co-ordinate to the eligibility criteria. On a 2d stage, full-texts of potentially relevant reviews were obtained for a final decision. Disagreements were resolved through give-and-take; if necessary, a third reviewer was consulted.
Information extraction of the included SRs
For included SRs, nosotros collected information about their general characteristics, as well every bit information about the gathered information from individual studies. For data extraction, each reviewer went through a pilot examination to standardize the process. We designed an extraction class to collect data at three levels:
Characteristics of included SRs and methodological quality
Nosotros collected data almost author(s), year of publication, search date, searched databases, objective, design, number of included studies and patients, and methods used for the assessment of take a chance of bias.
Two reviewers independently assessed the methodological quality of the included SRs by using the AMSTAR tool [17]. Disagreements were discussed until consensus was reached. We calculated a global AMSTAR score assigned i point for each particular rated every bit "yes" and items rated as "no"; "cannot reply", or "not applicable" obtained zippo points, resulting in an overall score ranging from 0 to 11. Based on the reported score we classified each SR into 3 categories: depression (0 to 3 points), moderate (4 to seven points), and loftier quality (8 to 11 points) [17].
Clinical questions assessed in the SRs
We collected information related to enquiry questions in PICO format (east.yard. Population, Intervention, Comparator and Outcomes). For descriptive purposes, we categorized conclusions reported by authors for each PICO question, into 6 categories: "unclear", "no effect", "probably harmful", "harmful", "probably beneficial" and "beneficial", as the categorization performed in previous prove mapping. See Table i, for further details of the category definition. Two reviewers independently categorized the conclusions. Discrepancies were discussed until consensus was reached. In all cases, sentence represented a formal assessment about the testify, benefits and harms of each intervention.
Characteristics of individual studies included in SRs
We collected the following data near the individual studies included in each SR: abstruse, number of included patients, land, funding, follow-up, type of written report, condition, intervention, comparison, and methodological quality according to the authors of the SRs.
Synthesize the results into a user-friendly format
We presented our findings on tables and figures to draw the characteristics of the included SRs. Additionality, we classified the data according to PICO questions. Thus, for each PICO we obtained the number of SRs, private studies, and patients.
We mapped the extent of the prove using bubble plots. Each bubble represents one SR. The chart displays information using iii dimensions: (i) Authors conclusions ("unclear", "no event", "probably harmful", "harmful", "probably benign" and "beneficial") in the x-axis; (ii) Score from AMSTAR assessment in the y-centrality, and (iii) The number of participants included in the SR assessing the PICO question represented in the chimera size. Systematic reviews may have been represented more than once in the plot as i SR could take answered different PICO questions.
Results
We obtained a full of 1323 records afterwards duplicates were removed. Following titles and abstracts screening, 93 articles were obtained in full-text for a final decision. We included a total of 44 SRs in the last selection (Fig. i). A list of excluded reviews with exclusion rationale is bachelor in Additional file 2.
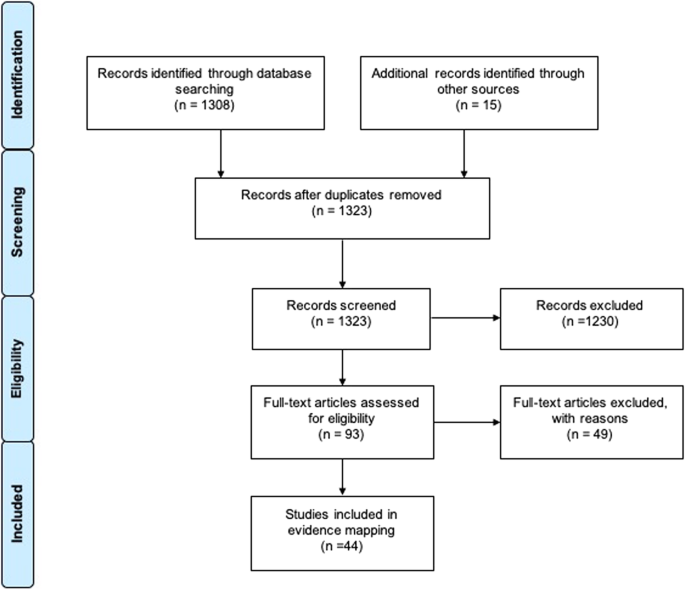
Flow chart outlining the study selection process
Characteristics and quality of systematic reviews
Included SRs were published between 2001 and 2017 comprising studies conducted betwixt 1975 and 2016. The last search was conducted in November 2016 [18]. All but ane SR assessed the effectiveness of cannabis or cannabinoids, while the remaining SR evaluated the cannabinoids adverse events only [19]. Seventeen out of 44 included SRs performed a meta-assay of data. Run across Table two for boosted characteristics of SRs.
Quality of the included SRs according to AMSTAR scores was categorized equally "low" in v studies (11,3%) [xx,21,22,23,24], as "moderate" in 22 studies [nineteen, 25,26,27,28,29,xxx,31,32,33,34,35,36,37,38,39,40,41,42,43,44] and as "high" in 17 SRs [ane, 45,46,47,48,49,50,51,52,53,54,55,56,57] (Fig. 2). The most frequent drawbacks of SRs included no reporting of conflicts of involvement, no assessment of publication bias, and absence of 'a priori pattern'.
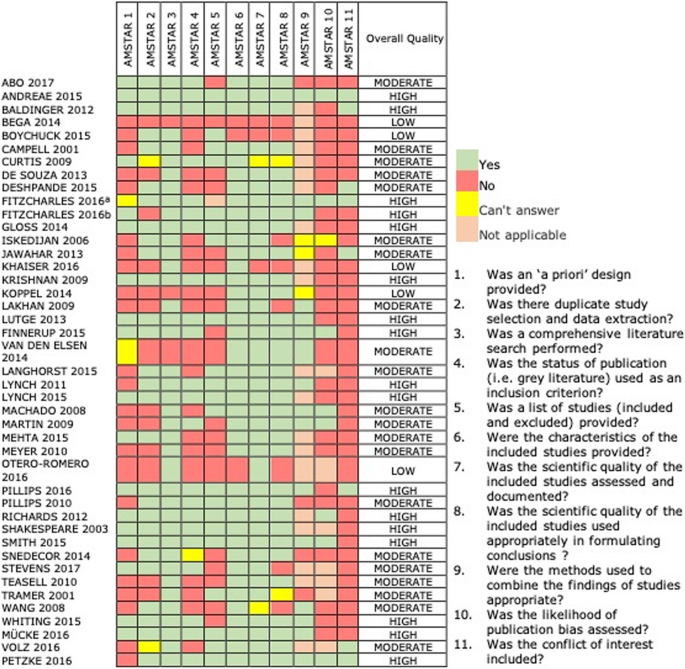
Methodological quality of included Systematic Reviews
Characteristics of individual studies
A full of 158 individual studies were analyzed in these SRs, after because duplication of studies. The number of included studies past review ranged from one [33, 39, 43, 46, 53, 58] to 79 [1]. One-hundred 40-six studies (92,four%) were randomized clinical trials (RCT), of which 59 were parallel and 84 were cantankerous-over trials, and the remaining three studies did not have enough information to define the type of RCT. Two studies (ane.2%) were not- randomized clinical trials (NRCT), vii (4,four%) were uncontrolled clinical trials, and 3 (one.9%) were observational studies.
Nigh of the private studies were conducted in the United states of america (northward = 57; 36%), followed by the Uk (due north = 29; 18,iii%), and Canada (n = 10; six,4%). Thirteen trials were conducted in more than i country (eight,3%). Xl-nine studies were funded by pharmaceuticals companies (31%) while 34 were funded by bookish societies (21,9%). Follow-up of participants ranged from 1 twenty-four hour period to 48 weeks. 1-hundred 15 studies compared interventions of cannabis or cannabinoids with placebo. Characteristics of individual studies are provided in Additional file 3.
PICO questions
We extracted 96 PICO questions. PICOs were grouped in the following clinical scenarios. We provided details of PICOs in Additional file 4.
Multiple sclerosis
The included SRs addressing the management of several symptoms associated to Multiple Sclerosis (MS), including pain, spasticity, bladder dysfunction, and tremor.
The largest number of SRs evaluated the effect of medical cannabis on MS related pain. When cannabinoids in general were compared with placebo, the authors of 2 SRs (15 RCT) claimed a "probably benign" and "unclear" conclusion, respectively [29, 44]. For this indication, in that location were four different cannabis presentations: oromucosal cannabis spray, oral cannabis' extract, smoked cannabis and dronabinol, all which were compared with placebo. Two SRs claimed "probably beneficial" [23] and "unclear" [50] conclusions for oromucosal cannabis spray. Oral cannabis' extract was assessed in one SR and the authors concluded a "beneficial" effect [23]. Ii SRs yielded an "unclear" conclusion for smoked cannabis [23, 28]. Finally, in three SRs where the presentation was dronabinol the authors concluded a "probably beneficial" decision [23, 29, 30]. Only ane SR assessed the efficacy of Nabilone plus Gabapentin versus an active control (Gabapentin lone), the conclusion was "probably benign" [50].
When investigating spasticity, cannabinoids were compared with placebo in three SRs (12 RCTs). The authors of ii SRs reported a "probably beneficial" determination [1, 31], and 1 an "unclear" conclusion [54]. Additionally, 3 administration routes were compared with placebo. Oral cannabis extract containing THC / CBD was examined in three SRs (five RCTs). The results of two SRs were reported as "probably beneficial" [23, 50], and in i the conclusion was "unclear" [24]. Oromucosal cannabis spray containing THC / CBD was studied in two SRs (five RCTs and ane uncontrolled trial), that reported a "probably benign" conclusions [23, 24]. Smoked cannabis was assessed in iii SRs (two RCTs), obtaining an "unclear" result [23], and "probably benign" conclusions [22, 50]. Finally, i SR compared THC-CBD (including both oral and oromucosal presentations) with THC solitary (iii RCT), its results were reported as "unclear" [31].
In relation to float dysfunction, an SR (two RCTs and ane NRCT) compared cannabis and placebo; authors concluded that cannabis is "probably beneficial" [18]. Specific presentations of cannabis were compared with placebo. Ane SR evaluated oromucosal cannabis spray (two RCTs), which was reported as "probably beneficial" [23]. In another SR, oral cannabis' extract was assessed in two different formulations: forms containing THC and CBD with data from five RCT, and forms containing THC alone with data from two RCTs. The conclusion for both comparisons was "no effect" [23].
Finally, 1 SR focused on cannabis' effect on tremor compared two formulations containing THC and CBD with placebo. 1 formulation was oral cannabis extract (three RCT), and the other was oromucosal cannabis spray (two RCT). For both comparisons, the conclusion was "no effect" [23] (Fig. 3).
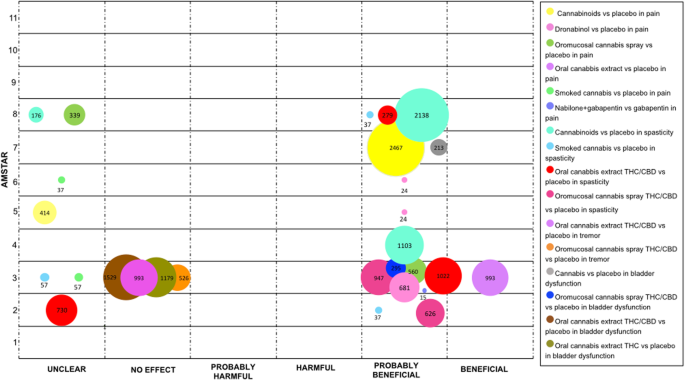
Evidence mapping of cannabis uses in Multiple Sclerosis
Movement disorders
Cannabinoids have been studied for symptomatic control of various involuntary motion conditions. In terms of Tourette Syndrome, iv SRs compared oral cannabinoid (dose 2.5–20 mg) versus placebo (2 RCTs), three of them ended that the furnishings were "unclear" and 1 as "probably beneficial" [1, 23, 26, 44].
Regarding Parkinson'south disease, one SR compared cannabis and cannabinoids versus placebo (2 RCTs) and reported an "unclear" conclusion [20]. For levodopa-induced dyskinesia, two SRs (one RCT) compared oral THC / CBD versus placebo and stated a "no effect" decision [23, 32].
For Huntington'southward illness cannabis was evaluated in one SR. This SR compared Nabilone and oral cannabidiol with placebo, each comparison included 1 RCT. For both comparisons, the authors concluded an "unclear" event [23].
Finally, the consequence of Dronabinol versus placebo for cramps in different atmospheric condition was evaluated. One SR (1 RCT) evaluated the effect in amyotrophic lateral sclerosis and some other SR (ane RCT) in Cervical Dystonia. Authors of both SRs reported an "unclear" effect [23, 46]. See Fig. iv.
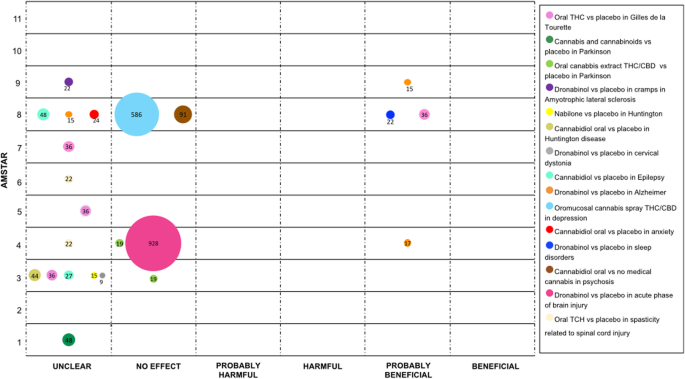
Evidence mapping of cannabis uses in Movement Disorders, psychiatric conditions and other neurological disorders
Psychiatric atmospheric condition
Psychiatric weather condition for which cannabis has been studied include: clinical depression, feet, sleeping disorders, and psychosis. Evaluating clinical depression, one SR (3 RCTs) compared oromucosal cannabis spray containing THC and CBD versus placebo, the authors determination was "no effect" [1].
For anxiety disorders, ane SR (one RCT), compared oral cannabidiol versus placebo, results were reported as "unclear" [1]. Also, for sleeping disorders, ane SR (one RCT) contrasted Dronabinol with placebo, showing a "probably beneficial" determination [1]. Finally, for psychosis one SR (two RCTs) evaluated oral cannabidiol versus no medical cannabis concluding as "no effect" [1]. See Fig. 4.
Other neurological disorders
The medical utilize of cannabis has been assessed in a heterogeneous group of neurologic conditions. Two SRs compared oral Cannabidiol with placebo for patients with epilepsy (three RCTs and one NRCT). Authors of both SRs obtained an "unclear" conclusion [23, 59].
Three SRs (two RCTs) evaluated Dronabinol compared with placebo to treat anorexia, disturbed behavior and agitation in patients with Alzheimer'south disease. The conclusions of the authors were "probably beneficial" in two SRs [32, 56] and "unclear" in one SR. [58]
In patients with an astute stage of acquired brain injury, Dronabinol was compared with placebo to manage intracranial pressure level. The comparing was assessed in one SR (two RCTs), and information technology was concluded that there is "no event" [37].
For the direction of spasticity in patients with spinal string injury, 2 SRs (one RCT) compared oral TCH with placebo. Authors concluded the effect to be "unclear" [36, 41]. Encounter Fig. iv.
Hurting in general
In that location is a big amount of evidence surrounding cannabis and cannabinoids in the management of astute and chronic pain. It has either been studied as an isolated symptom or in association with other diseases (i.east. diabetes mellitus or cancer).
Chronic pain
Two SRs (37 RCTs) assessed cannabis and cannabinoids with placebo. One SR reported "probably harmful" furnishings [35], and one SR reported a "probably beneficial" furnishings [1]. One SR evaluated cannabinoids versus codeine (two RCTs), results were reported to exist "probably harmful" [25]. Vaporized cannabis was also studied for chronic pain relief compared to placebo in one SR. A "probably benign" conclusion was establish in 21 patients involved in one uncontrolled study [22].
1 SR focused on the comparing of cannabinoids' effects confronting placebo for chronic pain, non associated with cancer. This review included ix RCTs for cannabis and cannabinoids, four RCTs for smoked cannabis, 7 RCTs for oromucosal cannabis spray and two RCTs for dronabinol. The conclusions for these four comparisons were stated as "beneficial" [l]. Furthermore, oral cannabis excerpt was compared with placebo in one SR, with two patients. The authors stated a "no effect" conclusion [25].
To consider the effects of cannabis and cannabinoids in neuropathic pain, four categories were established: neuropathic hurting in general, posttraumatic neuropathic pain, diabetic neuropathy, and neuropathic pain associated with allodynia.
With regards to neuropathic pain in general, three SRs compared cannabis and cannabinoids versus placebo (21 RCTs), conclusions were reported every bit "beneficial", "probably beneficial", and "unclear" [21, 44, 51]. When cannabinoids were compared with placebo three SRs were found (xv RCTs) [29, 49, 57], 2 SRs concluded that cannabinoids were "probably beneficial" and one as "probably harmful". When just cannabis was included in comparison, the authors of ane SR (4 RCTs) stated a "probably benign" determination [28]. Furthermore, smoked cannabis, vaporized cannabis, oromucosal cannabis spray and CT-iii (an analogue of THC-11-oic acrid) were compared with placebo, with "probably benign" conclusions for all of these comparisons.
Furthermore, two different cannabis presentations were compared with agile compounds in patients with neuropathic pain. One SR assessed oral cannabis extract versus codeine (ane crossover RCT with one patient), authors from this SR stated a "probably beneficial" conclusion [25]. 2 SRs (one RCT) that compare Nabilone with Dihydrocodeine stated conclusions considered as "no consequence" [51, 57].
In relation to doses, two SRs (iii RCTs) compared depression vs. high dosage of cannabis. Conclusions ranged from "probably beneficial" to "no effect" conclusions [22, 28].
For posttraumatic neuropathic pain, three comparisons were conducted. One SR evaluated smoked cannabis versus placebo (ane RCT), the decision was "unclear" [28]. Some other SR compared Dronabinol with Diphenhydramine (one RCT), the determination was "no effect" [36]. Regarding dose, one SR (ane RCT) compared depression vs. loftier dosage of cannabis, information technology was concluded as "unclear" [22].
For diabetic neuropathy, two cannabinoids presentations were compared with placebo. For Nabilone one SR ended as "probably beneficial" [l]. For cannabis spray (ane RCT), the obtained results were considered "unclear" by one SR. [39] In addition, one SR (ane RCT) compared high with low doses of vaporized THC, results were considered equally "probably beneficial" [22].
Finally, in patients with neuropathic pain associated with allodynia, one SR compared oromucosal cannabis spray with placebo, and ended every bit "probably beneficial" [50].
Acute hurting
Cannabinoids were compared with placebo in 1 SR (5 RCTs). The determination from the authors was "unclear" [40]. As well, Dronabinol and smoked cannabis were compared with placebo in one SR, with the inclusion of two and one RCT, respectively. The conclusion was rated past the authors as "probably beneficial" for Dronabinol and "unclear" for smoked cannabis [22]. The aforementioned SR, based on one RCT, evaluated smoked cannabis versus Dronabinol; it was concluded as "unclear" [22].
The postoperative etiology of acute pain was assessed in ii SRs, the intervention of Levonantradol was compared with placebo (2 RCTs). One of these SRs showed a "probably beneficial" decision [25], while the other presented an "unclear" determination [forty].
Two types of headaches were assessed in two SR. 1 SR, which included data of i observational report, contrasted cannabis with no medical cannabis for patients with migraine, a "probably beneficial" determination was obtained [22]. The other SR compared Nabilone versus placebo in patients with headache due to medication overuse. The conclusion was "probably beneficial" [50]. See Fig. 5.
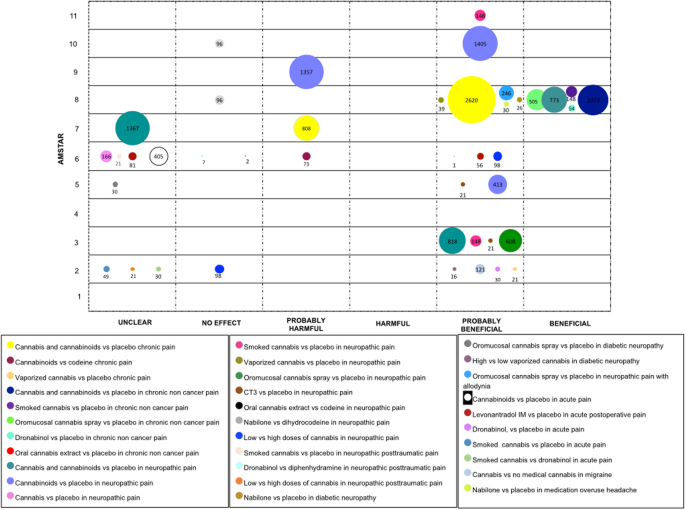
Show mapping of cannabis utilize in pain
Cancer
In patients with cancer, the most frequent symptom studied was emesis induced by chemotherapy, comparisons were performed with placebo and with active controls.
Four SRs compared cannabinoids with placebo (xiii RCTs) [ane, 42, 44, 55]. All these SRs stated a "probably beneficial" decision. One SR conducted a more specific comparison with Dronabinol versus placebo (three RCTs), its determination was stated as "unclear" [34].
Cannabinoids were compared with conventional antiemetics in vi SRs including a total of 31 RCTs [32, 34, 42, 44, 52, 55]. Two SRs obtained a "probably beneficial" conclusion, 2 an "unclear" decision, and two concluded equally "no effect". Meanwhile, one SR reported a "no effect" conclusion when compared cannabinoids plus antiemetic versus antiemetic lone (two RCTs and two NRCTs) [55].
2 SRs compared Dronabinol versus neuroleptics (4 RCTs), one of these SRs ended as "probably beneficial" and the other every bit "no consequence" [32, 34]. Nabilone and Levonantradol were compared with neuroleptics by ane SR (seven RCTs for Nabilone and two RCTs for Levonantradol) that reported an "unclear" conclusion [34].
In relation to anorexia associated with cancer, cannabinoids were compared with placebo in i SR (three RCTs), the conclusion was stated as "no effect" [56]. As well, in one SR Dronabinol was compared with Megestrol with data from one RCT, the decision was "probably harmful" effect [56].
Regarding cancer pain, one SR compared oral Benzopyranoperidine, oral THC and synthetic nitrogen analogue of THC with placebo. For Benzopyranoperidine, the conclusion from the authors was "no result" (1 RCT). For oral THC, the authors concluded every bit "probably beneficial" (ii RCTs). For synthetic nitrogen analogue of THC (two RCTs), the conclusion was reported every bit "probably harmful" [25].
This SR also compared the effects of cannabinoids confronting codeine. In ii studies, 1 RCT for Benzopyranoperidine and 1 RCT for oral THC, the authors ended "no outcome" in both comparisons. In regards to the synthetic nitrogen analogue of THC based on the information from one RCT, the conclusion was reported as "probably harmful". Additionally, constructed nitrogen analogue of THC was compared with secobarbital in i RCT; the decision was stated as "probably harmful" [25]. For refractory cancer pain, i SR concluded as "probably beneficial" when compared oromucosal cannabis spray with placebo (two RCT) [56].
Finally, two SRs evaluated oromucosal cannabis spray versus placebo for the management of chemotherapy induced neuropathic pain. These SRs with data from i RCT concluded equally "unclear" and "no issue [50, 57]. See Fig. 6.
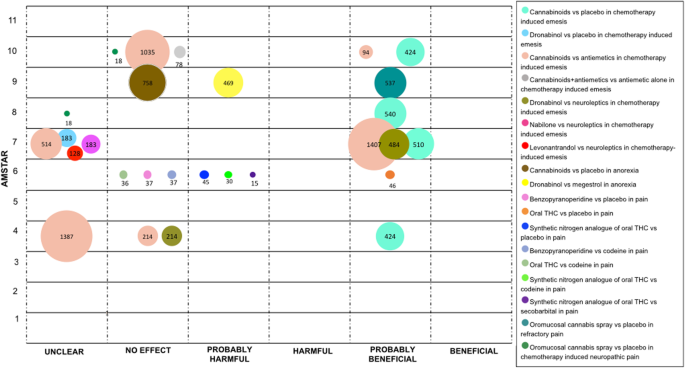
Evidence mapping of cannabis uses in Cancer
Other medical conditions
In this section, nosotros described several conditions which were not included in the previous sections.
The use of medical cannabis has been studied in rheumatic disorders such as rheumatoid arthritis, fibromyalgia, Crohn's illness, spinal chronic pain, and osteoarthritis. For rheumatoid arthritis, oromucosal cannabis spray was compared confronting placebo in 3 SRs (one RCT). Results were reported equally "probably harmful", "unclear", and "probably beneficial" [47, 48, 53]. For fibromyalgia two comparisons were conducted in four SRs [27, 47, 48, 51] with information from one RCT. When comparing Nabilone versus placebo and Nabilone versus Amitriptyline - three SRs concluded every bit "probably beneficial" and 1 equally "unclear" for the first comparison and the conclusions were "probably beneficial" in ii and "no upshot" in the other two SRs for the second comparison. In relation to Crohn's disease, two SRs compared smoked cannabis with placebo (one RCT), both studies concluded this intervention equally "probably beneficial" [33, 43]. For chronic spinal pain, one SR compared Nabilone with placebo (one RCT), its decision was reported as "unclear" [48]. Finally, for osteoarthritis of the genu, the PF-04457845, a fatty acid amide hydrolase-1 (FAAH1) inhibitor, was compared with placebo in ii SRs (one RCT), in both SRs, authors stated that there was "no effect" [47, fifty].
In patients with HIV-AIDS, cannabis and cannabinoids were compared with placebo for general symptoms in three SRs (eight RCTs), conclusions were "unclear" in two, and "probably beneficial" in ane [one, 44, 60]. For HIV-related neuropathic hurting, smoked cannabis was compared with placebo in three SRs (2 RCTs), the conclusions were "unclear" in ane and "probably beneficial" in two [22, 38, 51]. Regarding HIV wasting syndrome, three different comparisons were conducted. Herbal cannabis versus synthetic cannabinoids was addressed in 1 SR (i RCT), the conclusion was "unclear" [56]. Two SRs (five RCTs) compared Dronabinol with placebo and their conclusions were reported as "unclear" and "probably beneficial" [56, 60]. Dronabinol was also compared with Megestrol in i SR (i RCT) and the conclusion was "no upshot" [56].
Glaucoma was some other condition addressed in one SR (one RCT), where oromucosal cannabis spray was compared with placebo, the conclusion was reported every bit "unclear" [1].
Finally, in patients with chronic pulmonary obstructive illness, oromucosal cannabis spray was compared with placebo for the management of breathlessness in 1 SR (one RCT), the conclusion was "no result" [32]. See Fig. 7.
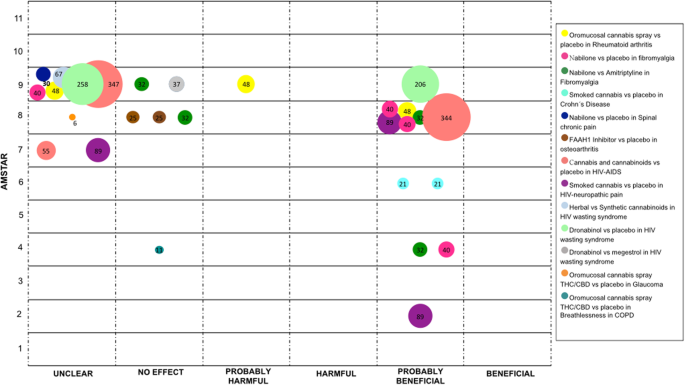
Evidence mapping of cannabis uses in other medical atmospheric condition
Discussion
Our prove mapping collected information from 44 SRs and 158 studies (most of them RCTs-92.4%) published between 2001 and 2017. The loftier number of studies reflects the increasing interest by users and physicians in assessing the potential therapeutic value of cannabis for several medical conditions.
We constitute that effectiveness and safety of medical cannabis has been evaluated in multiple medical weather such every bit multiple sclerosis, movement disorders (e.g. Tourette Syndrome, Parkinson Disease), psychiatric conditions, Alzheimer disease, epilepsy, acute and chronic hurting, cancer, neuropathic pain, symptoms related to cancer (e.g. emesis and anorexia related with chemotherapy), rheumatic disorders, HIV-related symptoms, glaucoma, and COPD.
Medical conditions addressed by these SRs have been previously identified by surveys about medical cannabis use [59,60,61,64]. I of the most representative surveys showed that cannabis was primarily used for back pain (eleven.9%), sleeping disorders (6.9%), depression (vi.seven%), pain resulting from injury or accidents (vi.2%), and multiple sclerosis (4.1%) [61].
However, we noticed that the evidence for medical cannabis effects on these conditions is heterogeneous regarding the conclusions and the quality of the nerveless studies. Most of the conclusions extracted from SRs were classified as "probably beneficial" and "unclear". Furthermore, for some comparisons, conclusions claimed past SRs were inconsistent and even contradictory. One example was the comparison of cannabis and cannabinoids with conventional antiemetics for chemotherapy-induced emesis [32, 34, 42, 44, 52, 55], where two SRs found a "probably beneficial" conclusions, while remaining four SRs claimed for an "unclear" conclusion or "no effect¨.
The testify supporting the medical use of cannabinoids varies widely by clinical scenarios from high to low quality bear witness. In fact, for some medical atmospheric condition, that nosotros found in this evidence map, studies cannot reach business firm conclusions, although RCTs take been conducted. While for other medical conditions, not showed in this prove mapping, cannabis has been canonical for use with only preliminary data (pre-clinical studies or observational studies) supporting the utilize, as is the case of hepatitis C, chronic renal failure, and posttraumatic stress disorders [65].
The research on health furnishings of cannabis and cannabinoids has been express by regulatory reasons and policies in some countries, leaving patients and health care professionals without the evidence to brand decisions regarding the use of cannabis and cannabinoids in local scenarios. Some barriers have been identified to conducting basic, clinical, and population health research on cannabis and cannabinoids, including regulations that restrict access to the cannabis products, funding limitations, and numerous methodological challenges [66].
In relation to funding, nosotros found that most of the analyzed individual studies were sponsored by pharmaceuticals companies. Because of complexity of the research agenda in this field more funding sources and mechanism are needed to better empathize the comprehensive wellness furnishings of cannabis.
There were also a number of methodologic limitations. The use of reliable placebos and well-selected active command compounds are needed for clinical trials, since the psychoactive and vasoactive furnishings of cannabis are a considerable challenge for effective blinding [66]. This limitation is important since 71% of the individual studies included in the SRs compared cannabis and cannabinoids against placebo.
Furthermore, restrictions on drug supply atomic number 82 to the lack of standardization in say-so or quantity of pharmacologically active constituents in cannabis products [66, 67]. This barrier leads to some other limitation in conducting clinical trials reflecting in the wide variety of cannabis compounds assessed for a given medical condition.
Moreover, to get well-validated bear witness it is necessary to accept high-quality research. The quality of the SRs was moderate to loftier according to AMSTAR scores. However, the most frequent drawbacks were: failure to declare conflicts of interest, lack of likelihood of publication bias evaluation and absenteeism of 'a priori design'. Additionally, it's important to country that beyond the quality of the SRs, its crucial to approximate the quality of the individual principal studies to go a context of what evidence is telling us.
1 strength of our evidence mapping is the use of a sensitive and comprehensive search strategy to localize the 44 SRs included as a source of information. Nosotros as well used a broad definition of SR in lodge to obtain the largest number of documents. Additionally, this evidence mapping uses a friendly format to organize and classify inquiry questions in PICO format. Findings are shown graphically to allow the identification of research needs, fields of controversy and the overall quality of the SRs included. Interventions were rated according to the conclusions stated by authors of the SRs. It is important to consider that this classification does not stand for the effect of the interventions.
One limitation of this evidence mapping is that the quality of the studies included in each SR was non evaluated in addition to the quality of the SRs. Furthermore, every bit it is a characteristic of bear witness mapping methodologies, we did not assess the quality of the evidence supporting the conclusions, which would have required the use of some complementary methodology such every bit GRADE. In add-on, were describe the conclusions of the included studies according to how the authors alleged them, nevertheless the management of effects for each comparison should be deeply assessed by systematic reviews. Despite these limitations, this bear witness mapping meets its objective of organizing and describing the available bear witness as reported past the authors.
Conclusions
In determination, the evidence on medical uses of cannabis is wide and highly heterogeneous. However, due to methodological limitations, conclusions were reported as "probably beneficial" and "unclear" in most of the assessed comparisons. To support the use of cannabis in different clinical atmospheric condition boosted efforts are needed, equally the approving for the utilize of cannabis and cannabinoids, equally any other drug, should rely on well-designed and statistically powered clinical trials.
Show mapping methodology is useful to perform an overview of bachelor enquiry, since it is possible to systematically describe the extent and distribution of evidence, and to organize scattered data. This approach helps to identify if there is plenty evidence to support policy maker's decisions, to recognize inquiry-dense areas where systematic reviews can be conducted, and to highlight enquiry priorities in the field. To attain these objectives, SRs are a reliable source of information as they convey comprehensive and appraised data. Furthermore, SRs help to expand or limit the scope of research mapping past modifying the search strategy according to the testify mapping aims.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- AMSTAR:
-
Assessing the methodological quality of systematic reviews
- CBD:
-
Cannabidiol
- COPD:
-
Chronic obstructive pulmonary disease
- FAAH1:
-
Fatty acrid amide hydrolase-ane
- HIV:
-
Human immunodeficiency virus
- MS:
-
Multiple Sclerosis
- NRCT:
-
Non-randomized controlled trial
- PICO:
-
Population, intervention, comparing, consequence
- RCT:
-
Randomized controlled trial
- SRs:
-
Systematic reviews
- THC:
-
Tetrahydrocannabinol
- UCT:
-
Uncontrolled trial
References
-
Whiting PF, Wolff RF, Deshpande S, Di NM, Duffy S, Hernandez AV, et al. Cannabinoids for medical use a systematic review and meta-assay. JAMA. 2015;313(24):2456–73.
-
Schrot RJ, Hubbard JR. Cannabinoids : Medical implications. Ann Med. 2016;48(3):128–41.
-
Bostwick JM. Blurred boundaries : the therapeutics and politics of medical marijuana. Mayo Clin Proc. 2012;87(2):172–86 Available from: https://doi.org/ten.1016/j.mayocp.2011.x.003.
-
Premoli M, Aria F, Bonini SA, Maccarinelli Thousand, Gianoncelli A, Della Pina S, Tambaro S, Memo G, Mastinu A. Cannabidiol: contempo advances and new insights for neuropsychiatric disorders treatment. Life Sci. 2019;224:120–7.
-
Kumar A, Premoli Thou, Aria F, Bonini SA, Maccarinelli G, Gianoncelli A, Memo M, Mastinu A. Cannabimimetic plants: are they new cannabinoidergic modulators? Planta. 2019. https://doi.org/ten.1007/s00425-019-03138-x.
-
Pisanti Southward, Bifulco Chiliad. Modern history of medical cannabis: from widespread use to prohibitionism and back. Trends Pharmacol Sci. 2017;38(3):195–eight Available from: https://doi.org/10.1016/j.tips.2016.12.002.
-
Volkow N, Baler R, Compton W, Weiss South. Agin wellness effects of marijuana use. N Engl J Med. 2014;370:2219–27.
-
Ciccone CD. Medical marijuana : just the start of a long, strange trip? Phys Ther. 2017;97(2):1–10.
-
Bifulco G, Pisanti Due south. Medicinal utilize of cannabis in Europe. EMBO Rep. 2015;16(2):130–2.
-
Aguilar BS, Gutiérrez V, Sánchez L, Nougier Chiliad. Medicinal cannabis policies and practices around the earth. Int Drug Policy Consort. Briefing paper. 2018;(April):1–32.
-
Lieberman MF. "Recredicinal" marijuana. Am J Ophthalmol. 2017;177:1–iv.
-
Bragge P, Clavisi O, Turner T, Tavender Eastward, Collie A, Gruen RL. The global evidence mapping initiative: scoping inquiry in broad topic areas. BMC Med Res Methodol. 2011;xi(ane):1–12.
-
Ballesteros M, Montero N, López-Pousa A, Urrútia G, Solà I, Rada G, et al. Evidence mapping based on systematic reviews of therapeutic interventions for gastrointestinal stromal tumors (GIST). BMC Med Res Methodol. 2017;17(i):135.
-
Arevalo-Rodriguez I, Muñoz Eastward, Buitrago-Garcia D, Nuñez-González S, Montero-Oleas Due north, Garzón V, et al. Quality assessment of controlled clinical trials published in orthopaedics and traumatology journals in Spanish: an observational study through handsearching and show mapping. SAGE Open up Med. 2018;half-dozen:1–9.
-
Ballesteros M, Montero N, López-Pousa A, Urrútia One thousand, Solà I, Rada G, et al. Prove mapping based on systematic reviews of therapeutic interventions for soft tissue sarcomas. Clin Transl Oncol. 2019;21(10):1398–1412.
-
Borgelt LM, Franson KL, Nussbaum AM, Wang GS. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy. 2013;22(2):195–209.
-
Shea BJ, Grimshaw JM, Wells GA, Boers Grand, Andersson N, Hamel C, et al. Development of AMSTAR : a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:1–seven.
-
Youssef NA, Schneider MP, Mordasini L, Ineichen BV, Bachmann LM, Chartier-kastler E, et al. Cannabinoids for treating neurogenic lower urinary tract dysfunction in patients with multiple sclerosis : a systematic review and meta-analysis. BJU Int. 2017;119:515–21.
-
Wang T, Collet J-P, Shapiro S, Ware 1000. Adverse furnishings of medical cannabinoids: a systematic review. C Tin Med Assoc J. 2008;178(thirteen):1669–78.
-
Bega D, Gonzalez-latapi P, Zadikoff C, Simuni T. A review of the clinical testify for complementary and alternative therapies in Parkinson's illness. Curr Treat Options Neurol. 2014;16(10):314.
-
Boychuk DG, States U, Paul S, Goddard G. The effectiveness of cannabinoids in the direction of chronic nonmalignant neuropathic pain: a systematic review. J Oral Facial Pain Headache. 2015;29(1):7–14.
-
Khaiser Chiliad, Pharmd C, Peng M, Pharmd C, Ahrari Southward, Mscphm C, et al. Medical cannabis dosing strategies in pain-related weather condition: a scoping review of electric current literature. J Hurting Manag. 2016;ix(4):449–63.
-
Koppel BS, Fife T, Youssof S. Systematic review : efficacy and rubber of medical marijuana in selected neurologic disorders. Report of the Guideline Evolution Subcommittee of the American University of Neurology. Neurology. 2014;82:1556–63.
-
Otero-Romero S, Sastre-Garriga J, Comi G, Hartung H, Sørensen PS, Thompson AJ, et al. Pharmacological management of spasticity in multiple sclerosis : systematic review and consensus paper. Mult Scler J. 2016;22(11):one–11.
-
Campbell FA, Tramèr MR, Carroll D, Reynolds DJM, Moore RA. Are cannabinoids an effective and safety handling choice in the direction of pain? A qualitative systematic review. BMJ. 2001;323:one–6.
-
Curtis A, Clarke C, Rickards H. Cannabinoids for Tourette ' s syndrome (review). Cochrane Database Syst Rev. 2009;(four):ane–13.
-
De Souza Due south, Desantana JM, Kenji F, Nogueira Ê, Lira da Silva D, Araújo-Júnior JX, et al. Efficacy and safety of medicinal plants or related natural products for fibromyalgia: a systematic review. Evidence-Based Complement Altern Med. 2013;2013:1–10.
-
Deshpande A, Mailis-gagnon A, Zoheiry N, Lakha Due south. Efficacy and adverse furnishings of medical marijuana for chronic noncancer pain. Can Fam Physician. 2015;61:372–81.
-
Iskedjian M, Bereza B, Gordon A. Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Curr Med Res Opin. 2007;23(1):17–24.
-
Jawahar R, Oh U, Yang S, Lapane KL. A systematic review of pharmacological pain management in multiple sclerosis. Drugs. 2013;73:1711–22.
-
Lakhan SE, Rowland G. Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: a systematic review. BMC Neurol. 2009;9(1):1–half dozen.
-
Van Den Elsen GA, Ahmed AIA, Lammers M, Kramers C, Verkes RJ, Van Der Marck MA, et al. Efficacy and safety of medical cannabinoids in older subjects: a systematic review. Ageing Res Rev. 2014;fourteen:56–64.
-
Langhorst J, Wulfert H, Lauche R. Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. J Crohn's Colitis. 2015;ix(1):86–106.
-
Machado F, Stefano Due south, De Cassia R, Rosa 50, Da Silveira D. Therapeutic use of Cannabis sativa on chemotherapy-induced nausea and vomiting amid cancer patients : systematic review and meta-analysis. Eur J Cancer Care (Engl). 2008;17:431–43.
-
Martín-Sánchez E, Furukawa TA, Taylor J, Martin JLR. Systematic review and meta-analysis of Cannabis treatment for chronic pain. Hurting Med. 2009;10(8):1353–68.
-
Mehta Due south, Mcintyre A, Janzen S, Loh Eastward, Teasell R. A systematic review of pharmacological treatments of pain after spinal string injury: an update. Arch Phys Med Rehabil. 2016;97(8):1381.
-
Meyer MJ, Megyesi J, Meythaler J, Murie-fernandez M, Aubut J, Foley N, et al. Acute management of acquired brain injury part Ii: an evidence-based review of pharmacological interventions. Brain Inj. 2010;24(5):706–21.
-
Phillips TJC, Cherry CL, Cox S, Marshall SJ, Rice ASC. Pharmacological treatment of painful HIV-associated sensory neuropathy: a systematic review and meta- analysis of randomised controlled trials. PLoS One. 2010;5(12):e14433.
-
Snedecor SJ, Sudharshan L, Cappelleri JC, Sadosky A, Mehta S, Botteman M. Systematic review and meta-analysis of pharmacological therapies for painful diabetic peripheral neuropathy. Hurting Pract. 2014;14(two):167–84.
-
Stevens AJ, Higgins Doctor. A systematic review of the analgesic efficacy of cannabinoid medications in the management of astute pain. Acta Anaesthesiol Scand. 2017;61(three):268–80.
-
Teasell RW, Mehta S, Aubut JL, Foulon B, Wolfe DL, Hsieh JTC, et al. A systematic review of pharmacological treatments of pain following spinal cord injury. Curvation Phys Med Rehabil. 2011;91(v):816–31.
-
Tramèr MR, Carroll D, Campbell FA, Reynolds DJM, Moore RA. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ. 2001;323(7303):16.
-
Volz M, Siegmund B, Hauser W. Efficacy, tolerability, and condom of cannabinoids in gastroenterology. A systematic review. Schmerz. 2016;30(1):37–46.
-
Amato L, Davoli M, Minozzi South, Mitrova Z, Parmelli Eastward, Saulle R, et al. Systematic reviews on therapeutic efficacy and safety of Cannabis (including extracts and tinctures) for patients with multiple sclerosis, chronic neuropathic pain, dementia and Tourette syndrome, HIV / AIDS, and cancer receiving chemotherapy. Epidemiol Prev 2017;41(5-6):279–293.
-
Andreae MH, Carter GM, Shaparin Due north, Suslov M, Ronald J, Ware MA, et al. Inhaled cannabis for chronic neuropathic hurting: an individual patient data meta-analysis. J Pain. 2016;xvi(12):1221–32.
-
Baldinger R, Hd K, Weber Thousand. Handling for cramps in amyotrophic lateral sclerosis/motor neuron illness (review). Cochrane Database Syst Rev. 2012;4:1–56
-
Fitzcharles M, Ste-marie PA, Auser WH, Clauw DJ, Jamal South, Karsh J, et al. Efficacy, tolerability , and safety of cannabinoid treatments in the rheumatic diseases : a systematic review of randomized controlled trials. Arthritis Care Res. 2016;68(5):681–8.
-
Fitzcharles MA, Baerwald C, Ablin JWH. Efficacy , tolerability and condom of cannabinoids in chronic pain associated with rheumatic diseases ( fibromyalgia syndrome , dorsum pain, osteoarthritis, rheumatoid arthritis ) a systematic review of randomized controlled trials. Der Schnerz. 2016;30(1):47–61.
-
Finnerup NB, Attal North, Haroutounian S, Moore A, Raja SN, Rice ASC. Pharmacotherapy for neuropathic pain in adults: systematic review, meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2016;14(ii):162–73.
-
Lynch ME, Ware MA. Cannabinoids for the treatment of chronic non-cancer pain: an updated systematic review of randomized controlled trials. J NeuroImmune Pharmacol. 2015;10(2):293–301.
-
Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72(5):735–44.
-
Phillips R, Friend A, Gibson F, Houghton E, Gopaul S, Jv C, et al. Antiemetic medication for prevention and handling of chemotherapy-induced nausea and airsickness in childhood (review). Cochrane Database Syst Rev. 2016;2:ane–94
-
Richards B, Whittle S, Buchbinder R. Neuromodulators for pain management in rheumatoid arthritis (review). Cochrane Database Syst Rev. 2012;1:one–51.
-
Shakespeare D, Boggild M, Ca Y. Anti-spasticity agents for multiple sclerosis (review). Cochrane Database Syst Rev. 2003;4:i–37.
-
Smith L, Azariah F, Lavender Five, Stoner Northward, Bettiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy (review). Cochrane Database Syst Rev. 2015;11:i–81.
-
Mucke Thou, Carter C, Cuhls H, Prub M, Radbruch L, Hauser W. Cannabinoide in der palliativen Versorgung Systematische. Schmerz. 2016;30(1):25–36.
-
Petzke F, Enax-Krumova E, Hause W. Wirksamkeit, Verträglichkeit und Sicherheit von Cannabinoiden bei neuropathischen Schmerzsyndromen. Schmerz. 2016;30(ane):62–88.
-
Krishnan S, Cairns R, Howard R. Cannabinoids for the treatment of dementia (review). Cochrane Database Syst Rev. 2009;2:1–xx.
-
Gloss D, Vickrey B. Cannabinoids for epilepsy (review). Cochrane Database Syst Rev. 2014;3:1–21.
-
Lutge E, Grey A, Siegfried N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS (review). Cochrane Database Syst Rev. 2013;4:1–35.
-
Arno H, Mark W, Kirsten M-V, Donald A, Franjo Chiliad. The medicinal use of cannabis and cannabinoids — an international cross-sectional survey on adiminstration forms. J Psychoactive Drugs. 2013;45(3):37–41.
-
Lucas P, Walsh Z. Medical cannabis access, utilize, and substitution for prescription opioids and other substances: a survey of authorized medical cannabis patients. Int J Drug Policy. 2017;42:30–5.
-
Sexton M, Cuttler C, Finnell JS, Mischley LK. A Cross-Exclusive Survey of Medical Cannabis Users: Patterns of Use and Perceived Efficacy. Cannabis Cannabinoid Res. 2016;1(i):131–viii.
-
Walsh Z, Callaway R, Belle-island L, Capler R, Kay R, Lucas P, et al. Cannabis for therapeutic purposes: Patient characteristics, admission, and reasons for apply. Int J Drug Policy. 2013;24(6):6–xi.
-
Loma KP. Medical Marijuana for Treatment of Chronic Pain and Other Medical and Psychiatric Problems A Clinical Review. JAMA. 2015;313(24):2474–83.
-
National Academies of Sciences, Engineering and Thousand. The Health Furnishings of Cannabis and Cannabinoids: The current state of evidence and recommendations for enquiry. The National Academies Press, editor. Washington, DC; 2017.
-
Bridgeman MB, Abazia DT. Medicinal Cannabis : History, Pharmacology, And Implications for the Acute Intendance Setting. Pharm Ther. 2017;42(3):180–8.
Acknowledgements
The authors would like to acknowledge Camila Montesinos-Guevara, Karol Quelal and Christopher Gault for their assist with the edition of this paper.
Funding
This work was supported past Universidad UTE. This funding source had no office in the blueprint, execution, analyses, interpretation of the data, or decision to submit results.
Writer information
Affiliations
Contributions
Conceived the study: NMO. Designed the study: NMO, SNG, DSR. Selection of studies: NMO, IAR, SNG. Extraction of data: NMO, IAR, SNG, AVG. Analyzed the information: NMO, SNG. Wrote the first draft of the manuscript: NMO, IAR, SNG, AVG, DSR. Contributed to the writing of the manuscript: NMO, SNG, AVG. Canonical the final manuscript and conclusions: NMO, IAR, SNG, AVG, DSR. Authors read and approved the last manuscript: NMO, IAR, SNG, AVG, DSR. The authors confirm the final article has been read and each writer's contribution has been approved by the advisable author.
Corresponding author
Ideals declarations
Ideals approval and consent to participate
"Not applicable".
Consent for publication
"Not applicable".
Competing interests
The authors declare that they take no competing interests.
Additional information
Publisher'southward Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Admission This article is distributed under the terms of the Artistic Commons Attribution 4.0 International License (http://creativecommons.org/licenses/past/4.0/), which permits unrestricted use, distribution, and reproduction in whatever medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and bespeak if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/i.0/) applies to the data made available in this article, unless otherwise stated.
Reprints and Permissions
About this article
Cite this article
Montero-Oleas, N., Arevalo-Rodriguez, I., Nuñez-González, South. et al. Therapeutic use of cannabis and cannabinoids: an prove mapping and appraisal of systematic reviews. BMC Complement Med Ther 20, 12 (2020). https://doi.org/10.1186/s12906-019-2803-2
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1186/s12906-019-2803-two
Keywords
- Cannabis
- Cannabinoids
- Medical marijuana
- Evidence mapping
- Evidence synthesis
Source: https://bmccomplementmedtherapies.biomedcentral.com/articles/10.1186/s12906-019-2803-2
0 Response to "Cannabinoids for Medical Use a Systematic Review and Meta-analysis"
Post a Comment